Nuvectis Pharma's NXP900: Breaking the Boundaries of Cancer Inhibition
Unmatched SRC/YES1 Potency & Selectivity — Amplified by a Transformative Type 1.5 Mechanism
Profound Breakthrough — NXP900, a best-in-class kinase inhibitor, delivers sustained, near-complete inhibition of SRC/YES1 cancer-driving signaling for the duration of therapy—setting a new standard in targeted cancer therapy.
Achieving high potency is critical for efficacy. Achieving high selectivity is critical for safety. These properties are often in conflict—improving one can compromise the other. However, there exist a few exceptional molecules that maximize both. NXP900 is one such molecule. It represents not only the best of kinase-targeting pharmacology but also introduces a transformative mechanism that magnifies its therapeutic profile even further.
Among the ~80 approved kinase inhibitors, NXP900 stands apart. It combines sub-nanomolar potency—among the most powerful ever developed—with exceptional selectivity, achieving full target engagement without dose-limiting toxicities.
Remarkably, NXP900 is the only kinase inhibitor shown to sustain peak-level inhibition of cancer-driving signaling continuously throughout the entire course of therapy. This level of control has not been achieved before and sets a new benchmark in kinase inhibition.
Two Pictures are Worth a Thousand Words
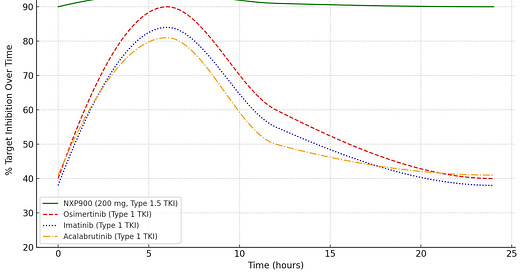
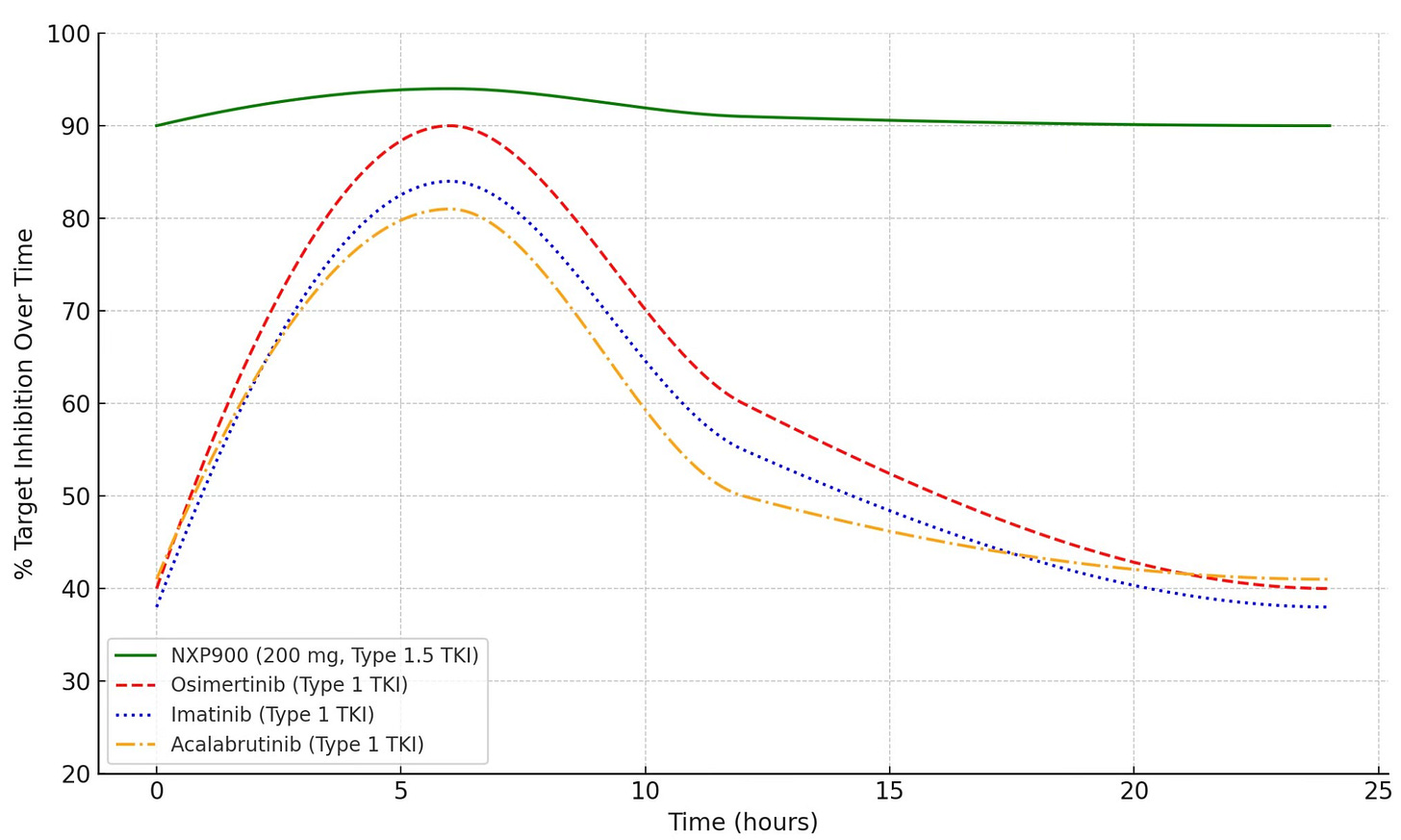
As shown in the graph below, NXP900 via its Type 1.5 mechanism maintains remarkably steady, peak-level inhibition of 90–95% throughout the entire 24-hour dosing interval (shown in green).
In contrast, leading blockbuster inhibitors such as osimertinib, which use conventional Type 1 mechanisms, rapidly lose activity as plasma levels decline between doses.
NXP900 vs. Top Blockbuster Kinase Inhibitors: % Target Inhibition Across a Single 24-Hr Dose Interval
Source: Truffle Pigs estimates based on available data.
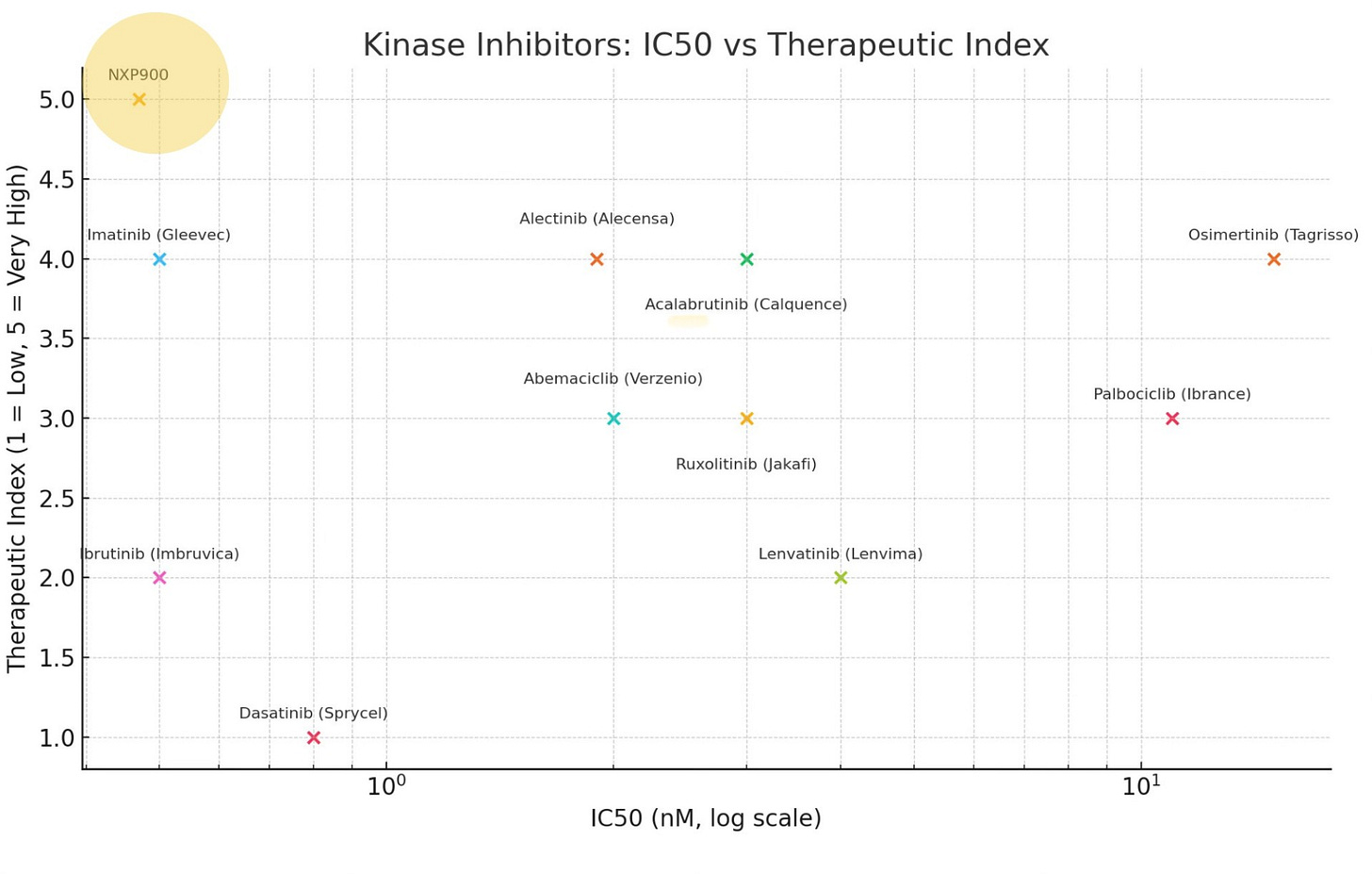
As demonstrated in the matrix below, NXP900 combines best-in-class potency with exceptional safety—surpassing all the top-performing kinase inhibitors developed to date.
Benchmark of Top Blockbuster Kinase Inhibitors: Therapeutic Index over Potency (IC50)
Source: Truffle Pigs estimates based on available data.
Investment Summary:
NXP900, a best-in-class SRC/YES1 tyrosine kinase inhibitor (TKI), represents the pinnacle of pharmacodynamic control. It has unmatched sub-nanomolar potency and exceptional selectivity, which is powered by a transformative Type 1.5 mechanism that delivers sustained, near-complete inhibition of cancer-driving signaling for the full duration of therapy—setting a new benchmark in targeted cancer treatment.
SRC-driven tumors represent an urgent unmet need, impacting over 100,000 U.S. patients annually, especially in large subsets of lung, head and neck, and breast cancers.
The first efficacy trials in target patients with SRC-driven tumors are set to begin within weeks, with initial data possible by year-end.
Now valued at $5 billion, Nuvalent (NUVL)—despite targeting a narrower segment of lung cancer—was already trading above a $1 billion market cap when it first released pharmacodynamic data. While those early results were promising, they pale in comparison to the unprecedented data just reported by NVCT—yet NVCT remains valued at just $200 million.
Meanwhile, investor capital continues to flood into the latest wave of Chinese me-too biotechs, often chasing modest, incremental improvements in crowded spaces. In stark contrast, NVCT is quietly driving a genuine leap forward in oncology with a truly first-in-class breakthrough—yet the market has yet to notice.
Best-In-Class SRC/YES1 Kinase Inhibitor
Super High Potency — NXP900 exhibits sub-nanomolar potency against YES1 (IC₅₀ = 0.47 nM) and low-nanomolar potency against SRC (IC₅₀ = 2.4 nM), placing it among the most potent TKIs ever developed.
Unmatched Selectivity — NXP900 demonstrates near-complete selectivity for SRC and YES1, offering the widest therapeutic window among all TKIs in its class.
Durable Type 1.5 Inhibition — Leveraging its Type 1.5 mechanism, NXP900 maintains remarkable, peak-level inhibition of 90–95% across the full 24-hour dosing interval—setting a new standard in targeted therapy.
Large Single-Agent Opportunity — SRC/YES1-driven tumors represent a significant unmet need, with over 50,000 new U.S. cases annually, primarily in lung cancer, head & neck cancers, and other solid tumors.
Strategic Combination Potential—SRC is a key resistance driver in many targeted therapies. NXP900 is ideally positioned for use in combination regimens, especially in EGFR- and ALK-driven NSCLC, where SRC/YES1 bypass pathways commonly emerge.
Type 1.5 Mechanism: Sustained Inactivation Beyond the Limits of Traditional TKIs
NXP900 isn’t just another TKI—it’s the first clinically validated SRC-family inhibitor to harness a rare Type 1.5 mechanism, locking SRC and YES1 in their native, inactive conformation. Unlike conventional Type 1 TKIs, which bind the active form and often fail to suppress non-catalytic scaffolding functions, NXP900 restores Tyr530-regulated auto-inhibition, the cell’s natural off-switch for SRC. This precision-based approach mirrors normal physiological regulation, effectively shutting down both catalytic activity and compensatory oncogenic scaffolding signaling at the source.
The result is a radical shift in inhibitory dynamics, delivering a level of sustained target suppression that conventional TKIs have never achieved. In contrast, leading blockbuster inhibitors such as osimertinib, which use conventional Type 1 mechanisms, rapidly lose activity as plasma levels decline between doses.
As shown in the graph above, NXP900 at 200 mg achieves sustained, near-complete inhibition of SRC/YES1 signaling, remarkably maintaining 90–95% suppression consistently across the full 24-hour dosing interval. This durable inhibition is independent of fluctuating plasma levels and is driven by NXP900’s Type 1.5 binding mechanism, which structurally inactivates the kinase. The result is a stable, uninterrupted shutdown of oncogenic SRC/YES1 signaling across every dose cycle.
Notably, the 250 mg single-dose cohort demonstrated even greater peak inhibition than 200 mg, suggesting that the steady-state inhibition at 250 mg may exceed the already impressive 90–95% range achieved at steady state for 200 mg. (Note: Steady-state data for the 250 mg dose was not available at the time of publication of this report.)
Conventional Type 1 inhibitors can also exhibit initial strong target inhibition for brief periods as plasma concentrations reach peak levels (Cmax) but show significant declines as drug levels fall toward trough, creating fluctuations in target suppression and windows of vulnerability for resistance. Take osimertinib, for example, a highly potent Type 1 TKI. While it achieves ~90% inhibition at Cmax, target suppression drops significantly between doses, sometimes falling as low as 40-50% at trough, resulting in mean inhibition that is well-below peak levels. These gaps allow residual signaling to persist, potentially driving resistance and tumor escape.
Peak Potency, Precision & Pharmacodynamic Control
NXP900’s combination of extraordinary sub-nanomolar potency and exceptional selectivity results in the widest therapeutic window of any TKI developed to date. On a matrix comparing IC₅₀ (potency) to therapeutic index (safety), NXP900 outperforms all top-selling TKIs, as illustrated above
NXP900’s sub-nanomolar potency against YES1 (IC₅₀ = 0.47 nM) is exceptionally rare, ranking NXP900 among the most potent kinase inhibitors ever developed. This level of potency enables complete target shutdown at clinically achievable doses.
NXP900 also demonstrates near-perfect target selectivity. In clinical studies, full plasma target engagement was achieved at doses as low as 150 mg, with no dose-limiting toxicities observed up to 250 mg—the highest dose tested. Simply put, NXP900’s full potency can be safely delivered to patients.
NXP900’s Type 1.5 mechanism sets it even further apart from the most advanced TKIs. By locking the kinase in its inactive conformation, NXP900 flattens the pharmacodynamic curve—sustaining a highly potent, peak level of inhibition across the entire dosing interval. This continuous suppression may lead to significantly improved therapeutic outcomes, enabling deeper, more consistent tumor control while minimizing opportunities for resistance.
Profound Implications — As a Type 1.5 inhibitor, NXP900 delivers an unprecedented pharmacodynamic profile, enabling deeper, more consistent target suppression with the potential for more durable, and even curative, responses in SRC/YES1-driven tumors.
A New Kind of Inhibition Comes to SRC: Type 1.5 > Type 1
The Type 1.5 mechanism may represent the only truly effective approach to inhibiting SRC and YES1—by targeting the kinases in their inactive conformation, blocking all oncogenic signaling and maintaining this suppression throughout the dosing interval.
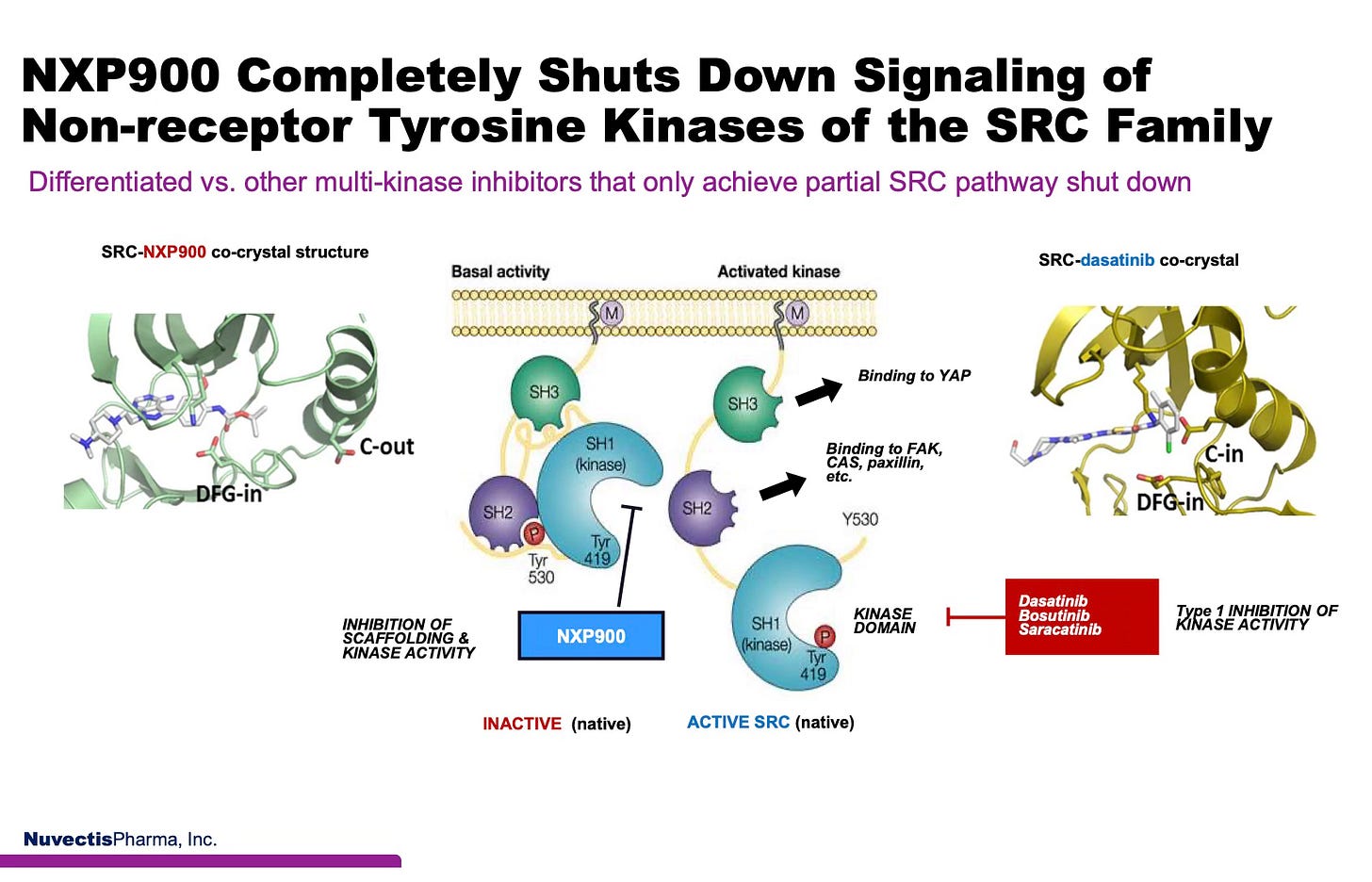
As illustrated in the center panel below, SRC’s native regulation relies on phosphorylation at Tyr530, which keeps the kinase folded into a closed, auto-inhibited, inactive conformation via its SH2 and SH3 domains. This is how SRC remains silent under normal physiological conditions. In cancer, however, this natural brake is disrupted, either through dephosphorylation or persistent upstream oncogenic signaling, resulting in the activation of SRC. Once active, SRC exposes Tyr419, enabling catalytic activity and scaffolding interactions with key oncogenic partners such as FAK, CAS, and paxillin, which drive tumor proliferation, metastasis, and resistance.
NXP900 is specifically designed to bind and stabilize SRC in its native, inactive conformation, restoring Tyr530-regulated auto-inhibition. As a true Type 1.5 inhibitor, NXP900 uniquely shuts down both catalytic activity and non-catalytic scaffolding functions of SRC and YES1. This sets it apart from earlier Type 1 inhibitors such as dasatinib and saracatinib (illustrated in the right panel), which bind SRC in its active form (C-in, DFG-in), inhibiting only kinase activity while allowing scaffolding-mediated signaling to persist.
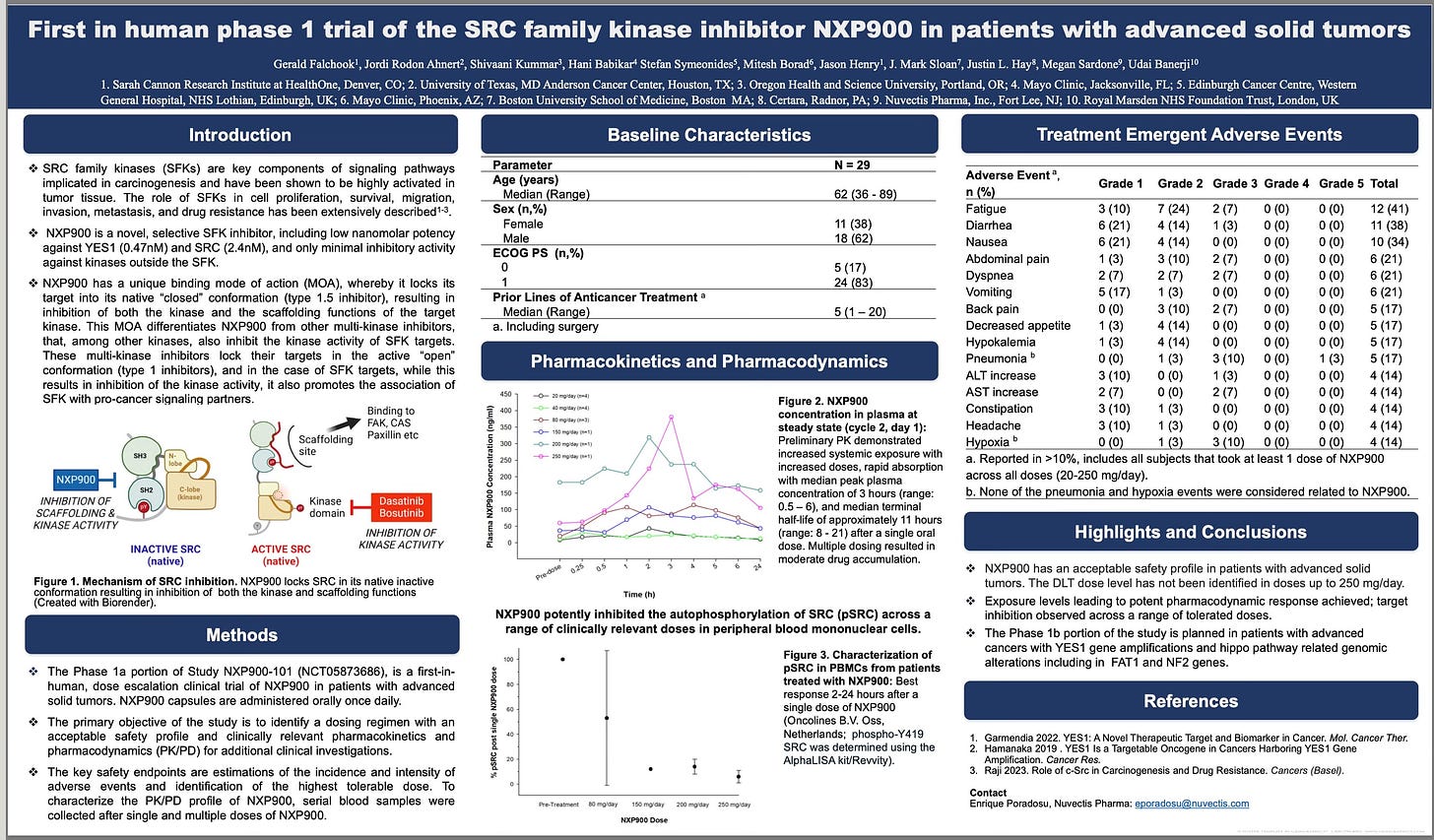
First-in-Human Phase 1 Study Demonstrates Exceptional Potency and Wide Therapeutic Window
The first-in-human Phase 1 trial of NXP900 in patients with advanced solid tumors provides early, compelling clinical validation of its novel Type 1.5 mechanism. NXP900 demonstrated high potency and selectivity, with sub-nanomolar activity against YES1 (IC₅₀ = 0.47 nM) and extremely low-nanomolar activity against SRC (IC₅₀ = 2.4 nM), while showing minimal off-target activity outside the SRC-family kinases.
Importantly, NXP900 exhibited a favorable safety profile: no dose-limiting toxicities were observed up to 250 mg once daily—the highest dose tested at that time. Reported adverse events were limited to mild gastrointestinal symptoms, which are common with oral cancer therapies, and fatigue in some patients. Pharmacokinetic data demonstrated predictable oral absorption and dose-proportional exposure, supporting the feasibility of once-daily dosing and sustained target engagement across the therapeutic range.
Source: First in human phase 1 trial of the SRC family kinase inhibitor NXP900 with advanced solid tumors, AACR 2025.
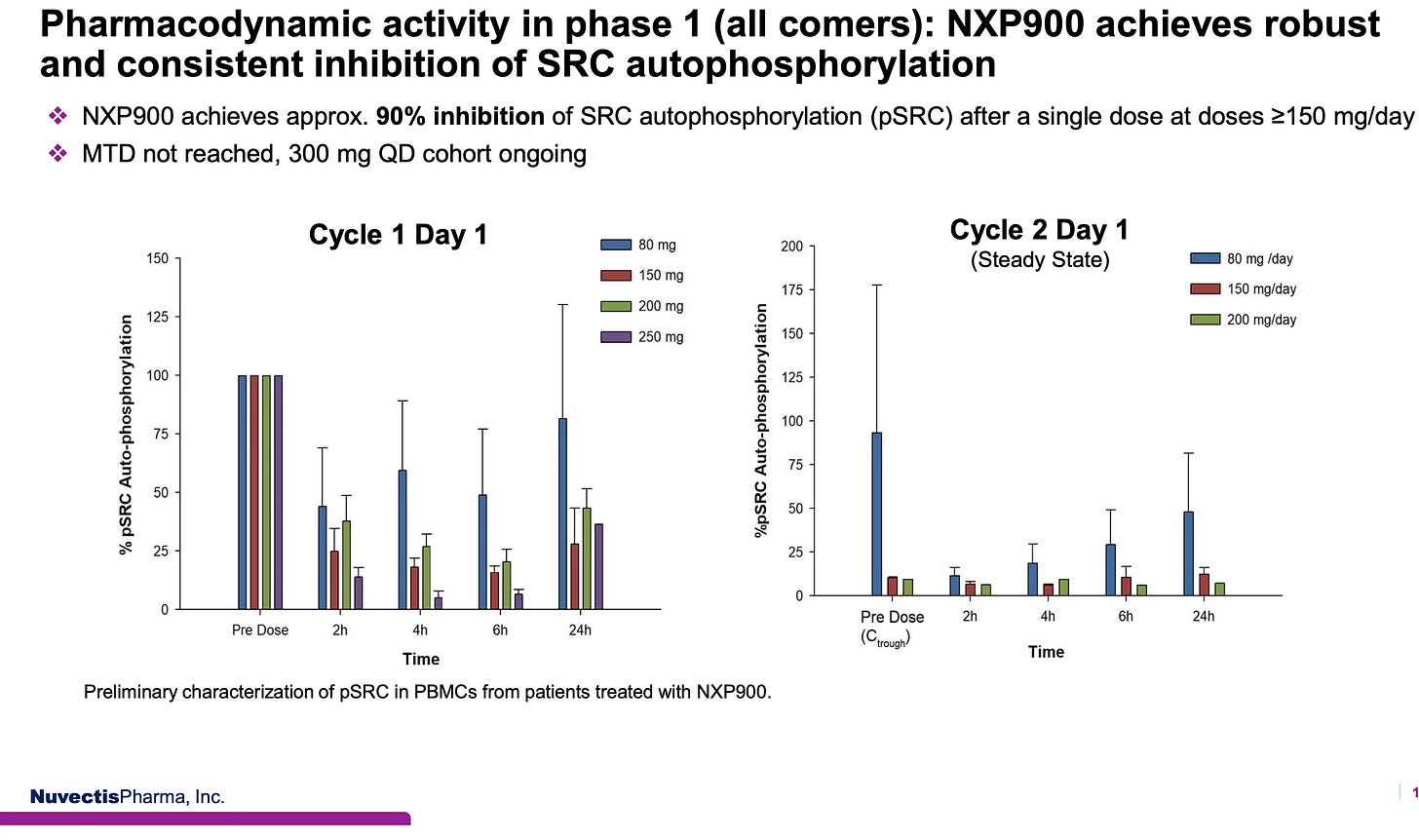
Remarkable Pharmacodynamics
But it is the exceptional pharmacodynamic (PD) results that offer critical insight into the Type 1.5 inactive-state binding mechanism, which illustrates the transformative potential of the molecule, as seen below:
Cycle 1, Day 1 (Initial Dosing)
In the graph on the left, % SRC auto-phosphorylation (pSRC) was measured at multiple time points following a single dose of NXP900. At doses > 150 mg, patients showed ~90-95% inhibition of SRC auto-phosphorylation within 2–6 hours. However, inhibition was cyclical, with some rebound by 24 hours, a pattern consistent with standard drug kinetics before reaching steady-state exposure.
Cycle 2, Day 1 (Steady State)
In the graph on the right, which shows data from Day 1 of Cycle 2, the PD results reveal something striking: at 150 mg and 200 mg, near-complete inhibition of SRC was observed even before the first dose of Cycle 2 was administered. This indicates that SRC remained locked in its inactive conformation throughout the prior 24-hour dosing interval, supporting a durable, steady-state restoration of auto-inhibition, the defining hallmark of a Type 1.5 mechanism.
This PD stability, unlike the on/off fluctuations typical of Type 1 inhibitors, suggests that NXP900 continuously locks SRC/YES1 in their natural silenced state, potentially disabling both enzymatic and scaffolding-driven cancer signaling for the full duration between doses of treatment.
Benchmark of SRC TKIs: Potency & Therapeutic Index
As shown in the chart below, NXP900 exhibits greater potency than Bristol Myers’ dasatinib, while achieving significantly higher selectivity, resulting in a much wider therapeutic window. In contrast, AstraZeneca’s saracatinib falls at the opposite end of the spectrum: although it offers improved safety over dasatinib, its weak potency against SRC leads to a poor overall therapeutic index.
Comparison of SRC Kinase Inhibitors: Therapeutic Index Over Potency
Source: Truffle Pigs estimates based on available data.
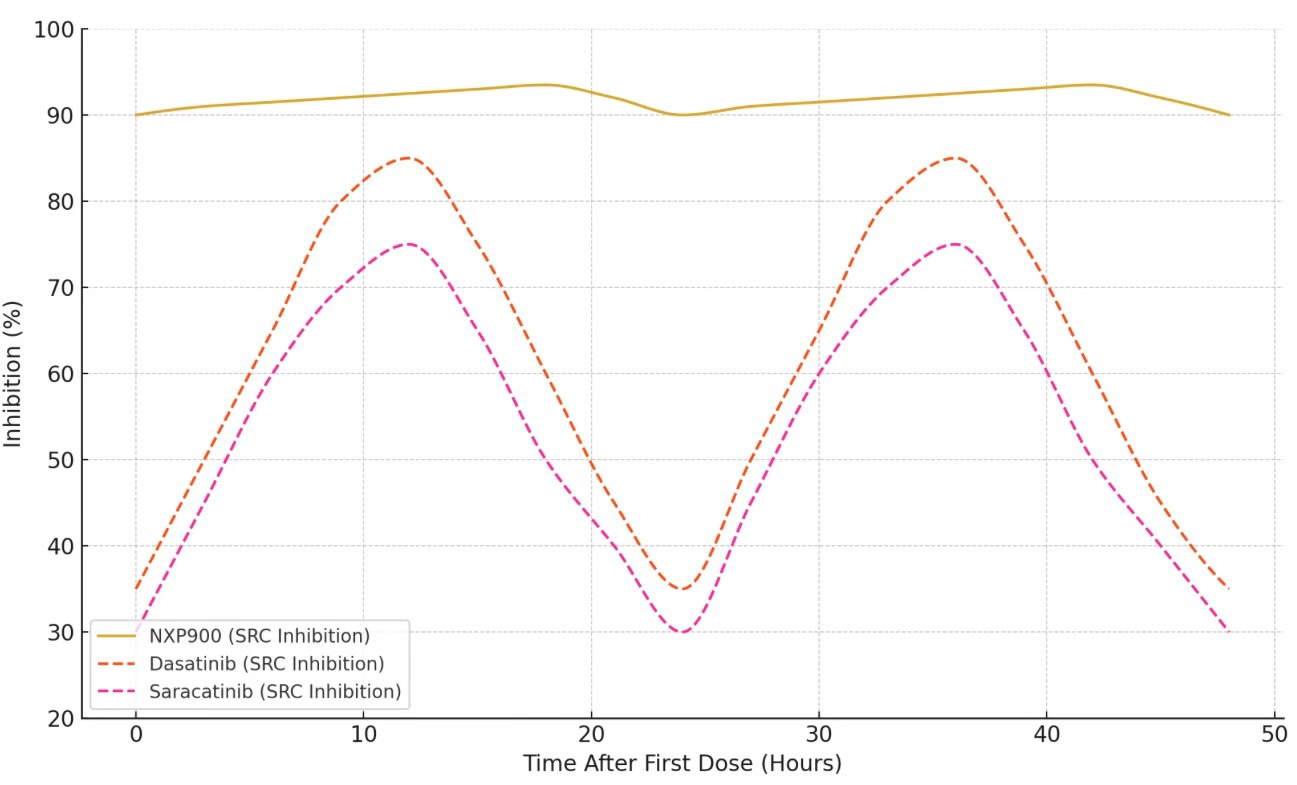
NXP900 Versus Legacy SRC TKIs: Dose Interval Pharmacodynamics
NXP900 is the only SRC-targeting TKI to utilize a Type 1.5 mechanism of action, while all legacy SRC inhibitors rely on conventional Type 1 mechanisms. As demonstrated, this mechanistic distinction drives dramatically superior pharmacodynamic performance: NXP900 achieves 90–95% mean target inhibition, compared to approximately 50-60% for dasatinib and even lower levels for saracatinib, as illustrated below.
SRC Type 1 & Type 1.5 Mechanisms: % SRC Inhibition Over Two 24-Hr Dosing Cycles (Steady State)
Source: Truffle Pigs estimates based on available data.
NXP900 > Legacy SRC Inhibitors in SRC-Driven Cancers
Legacy SRC inhibitors like dasatinib and saracatinib failed in solid tumors due to limitations in selectivity, potency, and mechanism of action, shortcomings directly addressed by NXP900.
Dasatinib, while potent against SRC, also inhibits ABL-BCR, severely compromising selectivity. This off-target activity restricts dosing due to dose-limiting toxicities, especially in solid tumors. In a notable case study, discussed below, a NSCLC patient treated with 60 mg dasatinib experienced a confirmed –69% tumor shrinkage, yet was forced to discontinue due to severe side effects.
Saracatinib offered better selectivity, but failed in the clinic due to very weak potency, as confirmed in its Phase 2 results, discussed below.
Both molecules also utilize Type 1 mechanisms, limiting their ability to fully shut down oncogenic scaffolding and achieve sustained inhibition.
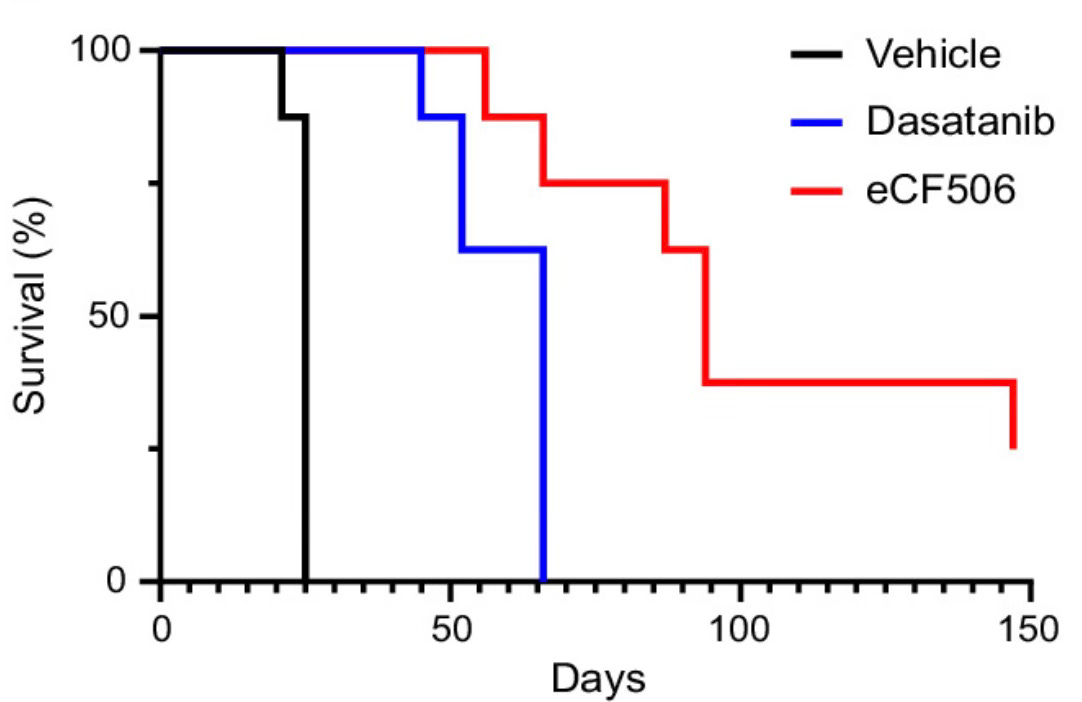
Head-To-Head Study: NXP900 & Dasatinib
In a head-to-head preclinical study of the two molecules, NXP900 outperformed dasatinib across all key endpoints:
Potency: NXP900 achieved full inhibition at 0.03–0.1 μmol/L, significantly outperforming dasatinib.
Efficacy: Both agents initially halted tumor growth, but dasatinib-treated tumors relapsed by day 35, 7 days after dosing concluded. In contrast, NXP900 sustained much longer tumor suppression, with 2/8 mice achieving complete response through day 148, the end of the study, and a third mouse surviving almost until the end of the study.
Tolerability: Despite being dosed 2x lower than NXP900, dasatinib showed cardiac toxicity, which caused heart enlargement and myocyte damage.
Head-to-head efficacy study between dasatinib and eCF506 (NXP900) in the primary breast cancer model in immunocompetent FVB mice (n = 8/group)
Source: A Conformation Selective Mode of Inhibiting SRC Improves Drug Efficacy and Tolerability, University of Edinburgh.
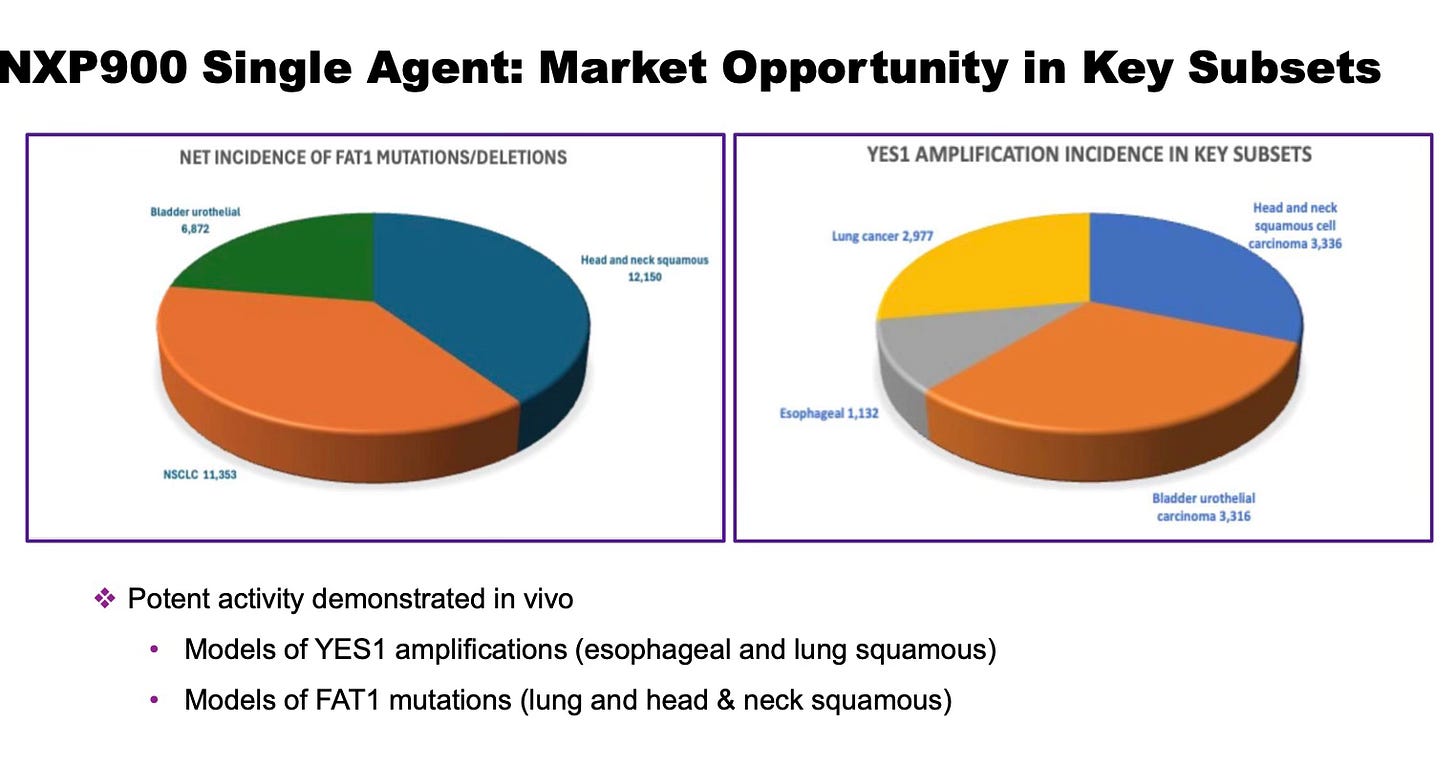
NXP900 Single-Agent Opportunities
SRC/YES1-driven tumors represent a significant unmet medical need, with an estimated annual incidence of more than 50,000 patients in the U.S. alone. This includes approximately 20,000 cases in non-small cell lung cancer (NSCLC, primarily squamous subtype), 15,000 in head and neck squamous cell carcinoma (HNSCC), and 10,000 in bladder urothelial carcinoma, among others. These tumors frequently harbor alterations in FAT1 and NF2, particularly in squamous histologies, and often exhibit amplification of YES1 and YAP1.
Nuvectis is advancing NXP900 into Phase 1B clinical trials across multiple SRC/YES1-driven indications, including NSCLC, kidney cancer, and mesothelioma. Additional study arms will include patients with HNSCC, bladder cancer, and esophageal cancer, among others.
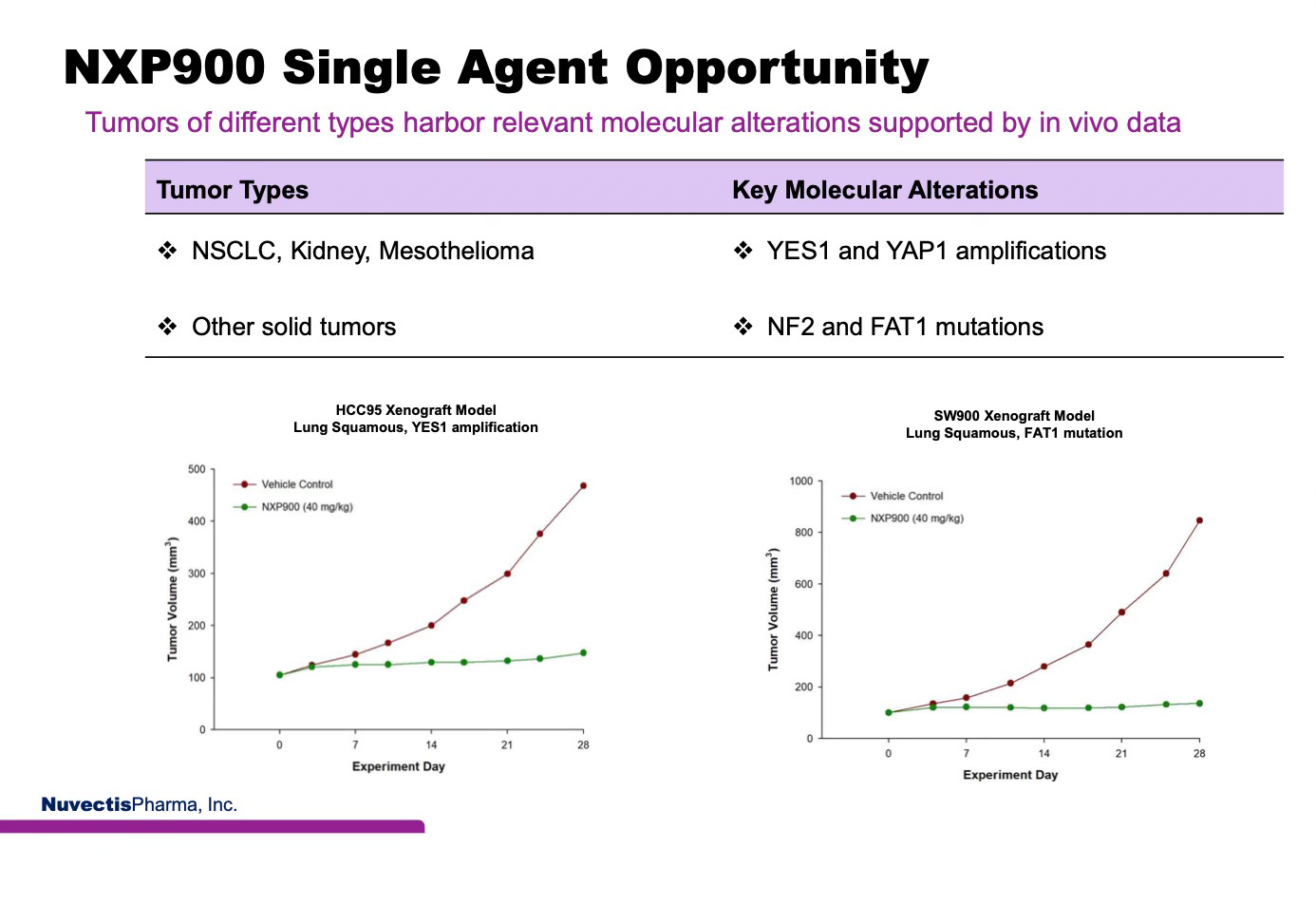
Strong Preclinical Evidence of NXP900 Efficacy in NSCLC
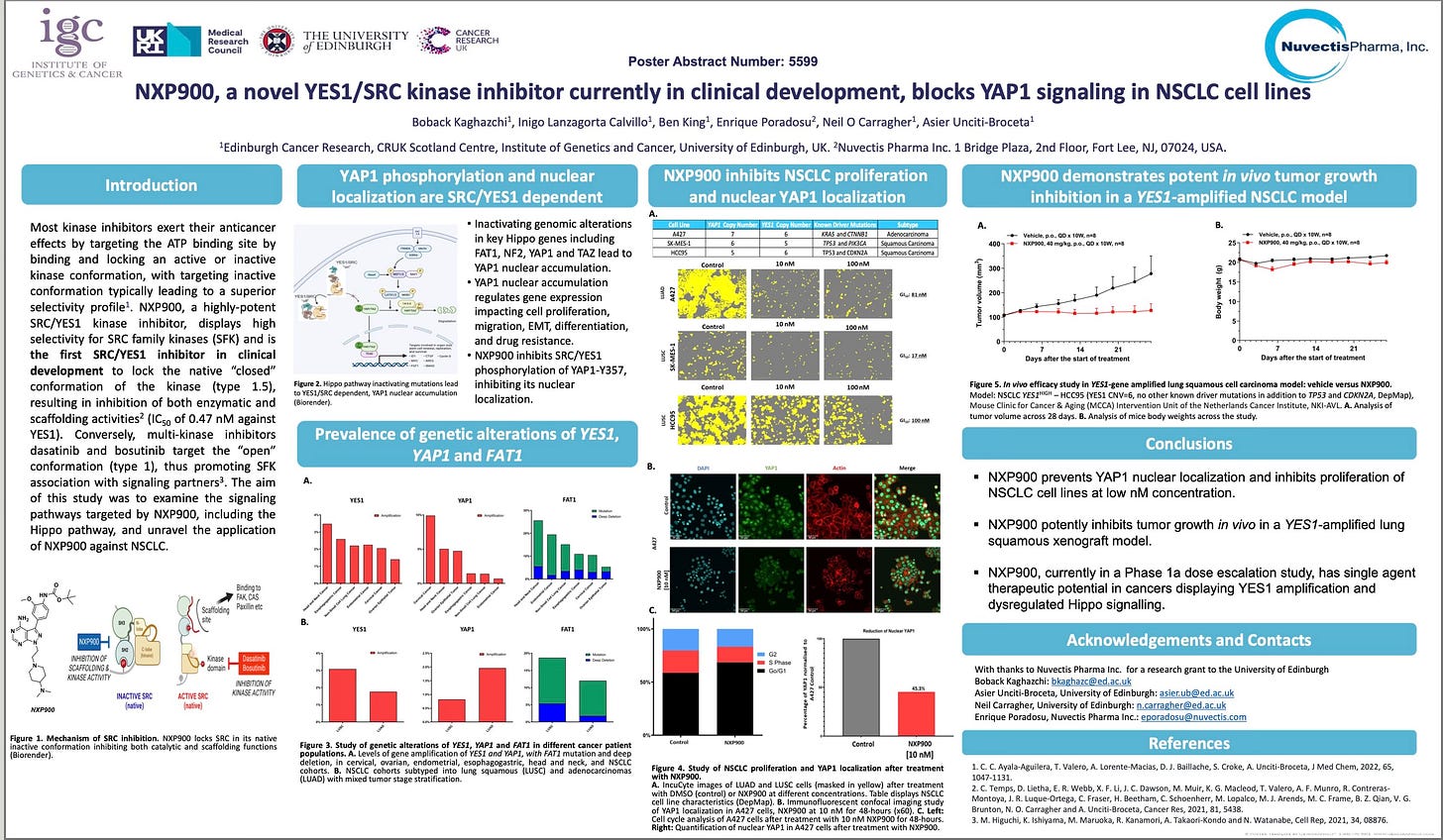
The charts and AACR poster below present preclinical in vivo models of squamous lung cancer. NXP900 demonstrates profound tumor control in models harboring YES1 amplifications and FAT1 mutations, showing marked efficacy relative to vehicle-treated controls.
Source: NXP900, a novel YES1/SRC kinase inhibitor currently in clinical development, blocks YAP1 signaling in NSCLC cell lines, AACR 2025.
Hints of NXP900 Efficacy in NSCLC: Case Study of Dasatinib
The case study below illustrates the single-agent efficacy of SRC inhibition in a patient with NSCLC adenocarcinoma, where YES1 gene amplification was the primary oncogenic driver. The patient—an 81-year-old male with stage IV lung adenocarcinoma and a 5.2-fold amplification of YES1—was treated with a low dose of dasatinib and achieved a confirmed partial response, with a deep 69% reduction in tumor size.
To validate these findings, Nuvectis conducted an in vivo study using the KYSE70 model, which harbors amplified YES1. As shown in the chart below, NXP900 induced profound tumor regression, further supporting YES1 amplification as a predictive biomarker for response. A link to the original abstract is provided at the end of this report.
Source: SRC Family Kinase Inhibition Targets YES1 and YAP1 as Primary Drivers of Lung Cancer and as Mediators of Acquired Resistance to ALK and Epidermal Growth Factor Receptor Inhibitors.
Hints of NXP900 Efficacy in NSCLC: Phase 2 Study of Saracatinib
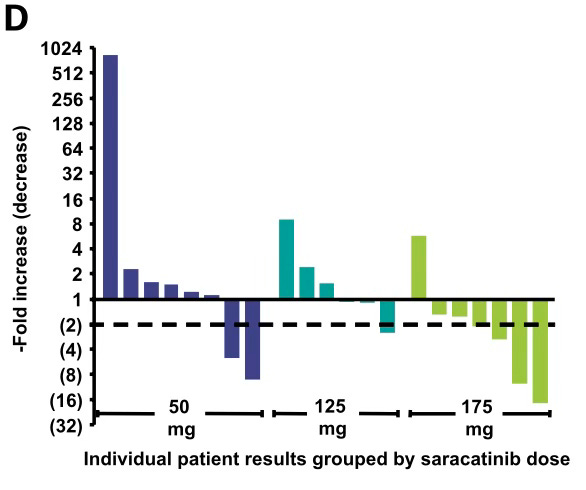
In 2014, AstraZeneca evaluated saracatinib, a first-generation Type 1 SRC inhibitor, in patients with advanced NSCLC, without selection for specific molecular alterations. At the recommended Phase 2 dose (RP2D) of 175 mg/day, saracatinib demonstrated limited clinical activity, including two partial responses lasting 3.7 and 14.6 months, and one case of stable disease with 29% tumor shrinkage sustained for at least four cycles, as shown in Figure 1 below.
However, pharmacodynamic studies revealed inconsistent SRC inhibition, with limited target suppression across the dosing interval. As shown in Figure D, only three of seven patients achieved >50% PD inhibition at the 175 mg dose, while three others showed <20% inhibition, including one patient with increased signaling activity.
This approximate 11% response rate (3 of 27 evaluable patients, including the case with 29% shrinkage) must be interpreted in the context of weak and variable PD performance, compounded by the absence of molecular enrichment. Notably, SRC/YES1 alterations are known to drive disease in approximately 10-15% of NSCLC cases.
In contrast, NXP900 has demonstrated markedly superior pharmacologic properties. Preclinical and early clinical studies show that NXP900 achieves consistent, sustained SRC inhibition exceeding 90% at doses >150 mg/day, with near-complete target suppression maintained across the full 24-hour dosing interval (AACR 2025). These findings, together with NXP900’s significantly higher potency and selectivity, highlight its differentiation from saracatinib and support a biomarker-driven strategy to optimize patient selection and maximize clinical benefit in NSCLC.
Source: Phase 2 trial of Saracatinib in NSCLC, 2014.
Saracatinib Phase 1 Pharmacodynamic (p-PAX/total PAX ratio)
Source: Phase 1 Study of Saracatinib in NSCLC, Clinical Cancer Res., 2010.
NXP900 Combination Opportunities: Acquired Resistance
The SRC bypass mechanism is a driver of acquired resistance across multiple targeted therapies, including EGFR and ALK inhibitors in NSCLC, enzalutamide in metastatic castration-resistant prostate cancer, and endocrine therapies in ER+ Luminal B breast cancer. As tumors evolve under therapeutic pressure, many reroute signaling through SRC/YES1 to maintain oncogenic activity.
To address this, Nuvectis is advancing NXP900 into Phase 1B combination studies with osimertinib and lorlatinib in EGFR-mutated and ALK fusion–positive NSCLC. In these patients, resistance frequently arises through activation of the SRC bypass pathway—now believed to be a dominant resistance mechanism.
Overcoming Osimertinib Resistance in NSCLC with NXP900
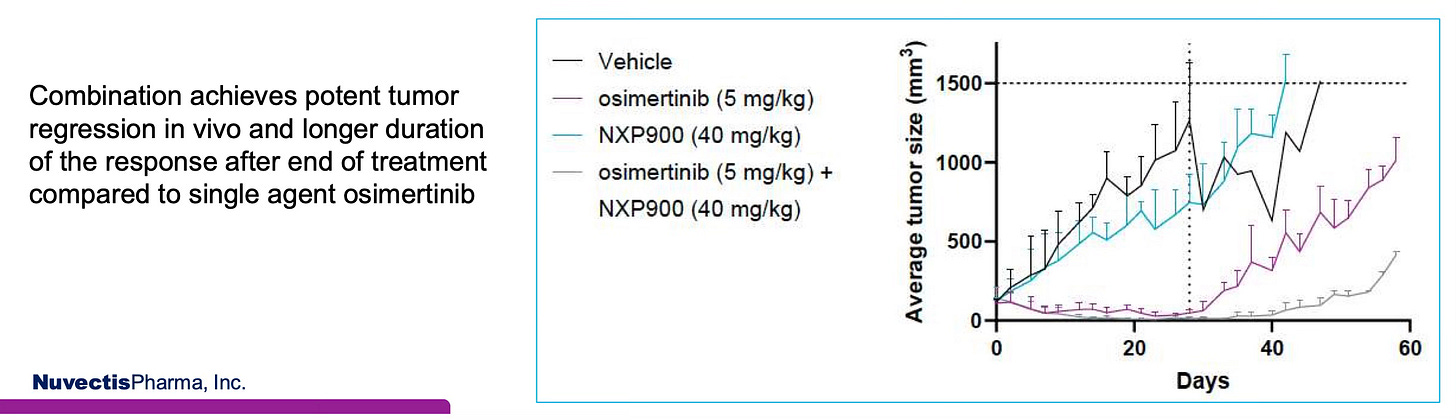
The potent, synergistic anti-tumor activity of the NXP900 and osimertinib (AstraZeneca’s Tagrisso) combination in NSCLC is clearly demonstrated in the chart from the AACR 2025 poster below. After 28 days of treatment, the combination resulted in greater tumor regression and significantly prolonged tumor control following treatment cessation—24 days versus just 4.5 days for osimertinib alone. Notably, the data suggest the possibility of a complete response in the combination arm, even after dosing ended at Day 28.
Given the central role of the SRC bypass pathway in EGFR-mediated resistance, these findings provide compelling rationale for NXP900 as a strategy to overcome or delay resistance and potentially extend the durability of response in EGFR-mutated NSCLC.
Preclinical In Vivo Study of NXP900 & Osimertinib
Source: Overcoming osimertinib resistance in NSCLC with NXP900, a phase 1, highly selective and potent first-in-class YES1/SRC inhibitor, AACR 2025.
Sources:
First in human phase 1 trial of NXP900 in patients with advanced solid tumors (AACR 2025)
Exploiting YES1-Driven EGFR Expression Improves the Efficacy of EGFR Inhibitors
Role of c-Src in Carcinogenesis and Drug Resistance
A Conformation Selective Mode of Inhibiting SRC Improves Efficacy and Tolerability
Dual targeting of RET and SRC synergizes in RET fusion-positive cancer cells
Macrophages promote anti-androgen resistance in prostate cancer bone disease
NXP800 publications can be found through this link: https://nuvectis.com/nxp800-publications/
NXP900 publications can be found through this link: https://nuvectis.com/nxp900-publications/
Risks of Investing in Development-Stage Biotech Stocks
Investing in development-stage biotech stocks carries significant risks that investors should carefully consider. These companies are typically focused on research and development of new therapies, drugs, or technologies, and their success is often contingent upon achieving regulatory approval, securing funding, and commercializing their products. Key risks include:
1 Regulatory Approval: The majority of development-stage biotech companies rely on successful clinical trials and subsequent approval from regulatory bodies such as the FDA. Failure to meet clinical endpoints or obtain regulatory approval can lead to significant financial losses.
2 High Failure Rates: Many therapies and drugs in development never reach the market due to safety concerns, lack of efficacy, or other unforeseen challenges.
3 Capital Intensity: Developing biotechnology products is costly, and these companies often depend on external financing. Dilution of existing shareholders through equity offerings is common.
4 Market Competition: Even if a product is successfully developed, it may face intense competition from other biotech firms or larger pharmaceutical companies with more resources.
5 Uncertain Revenue: Until a product is approved and commercialized, development-stage biotech companies often have little to no revenue, making their valuation speculative and highly volatile.
6 Economic and Market Conditions: Broader market or economic downturns can disproportionately impact biotech stocks, especially those reliant on high-risk capital.
Investors should thoroughly research and understand the unique risks associated with individual companies and consider their own risk tolerance before investing in development-stage biotech stocks.
Disclosure Statement
This report on Nuvectis Pharma Inc has been prepared solely for informational purposes. We confirm that we are not being compensated for the preparation or dissemination of this report. Furthermore, we currently own shares of NVCT. Please note that we are under no obligation to provide updates to the market regarding the sale or purchase of NVCT shares in the future.
We are not registered investment advisers, and this report does not constitute investment advice. Additionally, we do not make any representations or warranties regarding the accuracy, completeness, or reliability of the information contained herein. Readers are encouraged to conduct their own due diligence and consult with a qualified financial professional before making any investment decisions. We believe that this disclosure is made in compliance with applicable regulations and to provide transparency about our position in the subject of this report.