Nuvectis Pharma's NXP900: The First Cancer Inactivator
More than a targeted inhibitor—NXP900 doesn’t just inhibit cancer, it shuts it down.
NXP900 isn’t just a next-gen SRC kinase inhibitor—it’s the first SRC kinase inactivator. Unlike traditional SRC inhibitors, NXP900 fully shuts down all cancer signaling and keeps it off between doses. This marks a breakthrough in precision oncology, with transformative potential in lung cancer—the difference between simply extending life and potentially delivering the first durable cure.
Why It Matters
First-in-class SRC kinase inactivator: As a rare type 1.5 inhibitor, NXP900 locks SRC in its inactive state—eliminating all kinase activity, including from pro-cancer signaling partners.
Durable inactivation: Unlike the other SRC drugs that only inhibit some signaling, NXP900 maintains continuous, full shutdown of the cancer driver—delivering around-the-clock blockade that opens the door to durable responses—or even cure.
Exceptional selectivity: NXP900 precisely targets SRC with minimal off-target activity, enabling safe combination with other cancer therapies.
Large potential in lung cancer: Most lung cancers treated with targeted agents develop resistance via a SRC-driven bypass mechanism—making NXP900 an ideal combo agent for SRC-driven tumors, which are common in nearly half of the 200,000 lung cancers annually.
Achieving super high selectivity and complete inactivation of the target kinase are each very rare and to have both in a single molecule is an extremely powerful combination.
NXP900 First In Human Clinical Data
We attended AACR 2025, where the NXP900 poster delivered exactly what we hoped to see—clinical evidence of exceptional selectivity and full, sustained SRC kinase inactivation.
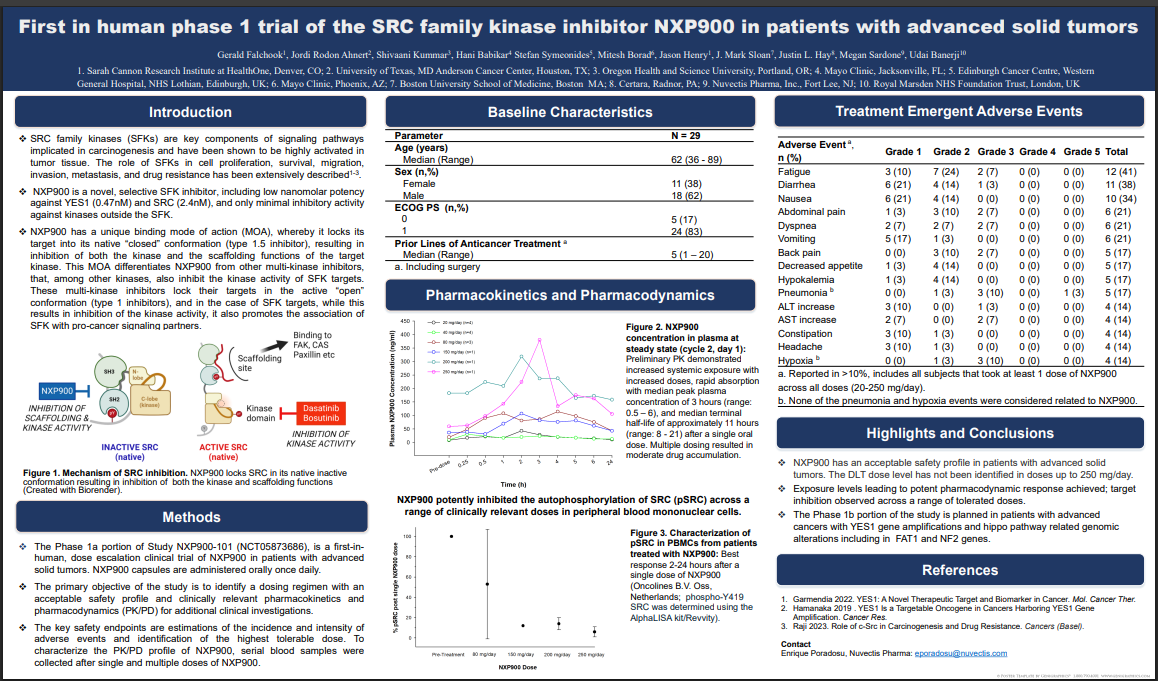
As can be seen in the poster below, NXP900 demonstrated a favorable safety profile, with no drug-related dose-limiting toxicities, no dose reductions, and no tolerability issues up to 250 mg. Safety issues were mainly GI-related, common to oral cancer drugs.
Pharmacokinetics showed dose-dependent exposure, rapid absorption, and moderate accumulation with repeated dosing. Pharmacodynamics (PD) showed dose-dependent inhibition across the range of doses tested (20-250 mg/day).
Importantly, PD data showed highly potent and sustained inhibition of SRC (90–95%) at doses as low as 150 mg and up to 250 mg, indicating exceptionally strong on-target activity and a wide therapeutic window.
The clinical data from this non-targeted all-comers study, along with supporting preclinical studies, provide a strong rationale for selecting patients with YES1 amplification and Hippo pathway alterations in the upcoming Phase 1B study—and bodes well for efficacy in targeted patients.
See encouraging comments at the end of this report from two researchers we met at AACR.
Where It Works
1. Single-Agent Opportunity: SRC/YES1-Driven Tumors
Certain lung cancers are primarily driven by SRC/YES1 or related genomic alterations like FAT1. These cancers are biologically "addicted" to SRC/YES1 signaling. By inactivating SRC, NXP900 has the potential to shut off their fuel source entirely—offering a new, precision-targeted treatment option. SRC/YES1 tumors are an urgent unmet medical need and represent an estimated 20,000 lung cancer patients annually.
2. Combination Opportunity: Acquired Resistance
Even more compelling is the opportunity in lung cancer patients who develop acquired resistance to targeted drugs (TKI) like AstraZeneca’s osimertinib (EGFR) or Pfizer’s lorlatinib (ALK). When TKI-treated lung cancers relapse, they often reroute through the SRC bypass mechanism. The SRC bypass pathway is so dominant in these patients, that some leading cancer researchers see NXP900 with potential to be the first durable cure for a significant number of patients.
This makes NXP900 a potential foundational therapy for a broad range of targeted combinations in lung cancer, comprising the vast majority of non-small cell lung cancer (NSCLC) subtypes. This includes well-studied targets such as EGFR and ALK, as well as emerging targets like KRAS, BRAF, MET, HER2, RET, and ROS1, where accumulating evidence suggests that activation of SRC bypass pathways contributes to acquired resistance. These NSCLC subtypes total around 70,000 new patients annually.
Overcoming Osimertinib Resistance in NSCLC with NXP900
The potent synergistic anti-tumor activity of NXP900 + osimertinib (Tagrisso) combo in NSCLC can clearly be seen in the AACR 2025 poster below. After 28 days of dosing, the combo showed greater tumor regression and significantly longer tumor control after treatment was ended—24 days versus 4.5 days for osimertinib alone. If you look closely at the data, you may even see hints of a complete response for the combo as dosing is discontinued at day 28.
Given the dominant role of the SRC bypass pathway in EGFR acquired resistance, there is strong rationale to believe that NXP900 could be highly effective in reversing resistance.
What Researchers/Scientists Say
One key opinion leader we met at AACR said that even with good targeted agents like osimertinib, complete responses are seen in only 2–3% of patients due to residual tumor cells that remain and later drive resistance. NXP900’s potent SRC inhibition may eliminate these persister cells, potentially making it the first targeted agent to meaningfully increase cure rates.
Another researcher said that since the SRC bypass pathway plays such a dominant role in EGFR and ALK acquired resistance, he’s very confident NXP900 will be highly effective in reversing the resistance—he believed the probability of success to be close to 100%.
What It Means For NVCT
Nuvalent (NUVL) reached a $5 billion valuation based on Phase 1 clinical response data in ALK and ROS1 lung cancers—patient populations totaling around 10,000 annually. Tagrisso, AstraZeneca’s top-selling drug, generates sales of nearly $7 billion annually targeting EGFR mutations representing 20,000 new patients annually. In contrast, Nuvectis’ NXP900 targets a much broader potential of about 90,000 lung cancer patients, with clinical response data anticipated later this year.
We estimate NXP900 peak sales of $12-15 billion from lung cancer single agent and combos, as well as single agent opportunities in head and neck, colorectal and esophageal cancers, among others.
Price Target: NVCT has only 23 million shares outstanding, so the simple math regarding potential upside to the share price is just too crazy for us to put into print.
First in human phase 1 trial of NXP900 in patients with advanced solid tumors (AACR 2025)
Risks of Investing in Development-Stage Biotech Stocks
Investing in development-stage biotech stocks carries significant risks that investors should carefully consider. These companies are typically focused on research and development of new therapies, drugs, or technologies, and their success is often contingent upon achieving regulatory approval, securing funding, and commercializing their products. Key risks include:
1 Regulatory Approval: The majority of development-stage biotech companies rely on successful clinical trials and subsequent approval from regulatory bodies such as the FDA. Failure to meet clinical endpoints or obtain regulatory approval can lead to significant financial losses.
2 High Failure Rates: Many therapies and drugs in development never reach the market due to safety concerns, lack of efficacy, or other unforeseen challenges.
3 Capital Intensity: Developing biotechnology products is costly, and these companies often depend on external financing. Dilution of existing shareholders through equity offerings is common.
4 Market Competition: Even if a product is successfully developed, it may face intense competition from other biotech firms or larger pharmaceutical companies with more resources.
5 Uncertain Revenue: Until a product is approved and commercialized, development-stage biotech companies often have little to no revenue, making their valuation speculative and highly volatile.
6 Economic and Market Conditions: Broader market or economic downturns can disproportionately impact biotech stocks, especially those reliant on high-risk capital.
Investors should thoroughly research and understand the unique risks associated with individual companies and consider their own risk tolerance before investing in development-stage biotech stocks.
Disclosure Statement
This report on Nuvectis Pharma Inc has been prepared solely for informational purposes. We confirm that we are not being compensated for the preparation or dissemination of this report. Furthermore, we currently own shares of NVCT. Please note that we are under no obligation to provide updates to the market regarding the sale or purchase of NVCT shares in the future.
We are not registered investment advisers, and this report does not constitute investment advice. Additionally, we do not make any representations or warranties regarding the accuracy, completeness, or reliability of the information contained herein. Readers are encouraged to conduct their own due diligence and consult with a qualified financial professional before making any investment decisions. We believe that this disclosure is made in compliance with applicable regulations and to provide transparency about our position in the subject of this report.